| Home | AmMin | GMR | RiMG | Collectors Corner | Directory | Short Courses | |
|
|
|||||||

|
|
Volume 26, pages 429-436, 1941 CRYSTALLOGRAPHIC NOTES: CAHNITE, STOLZITE, ZINCITE, ULTRABASITE CHARLES PALACHE, Harvard University, Cambridge, Massachusetts.* CRYSTAL CLASS OF CAHNITE
In the first description of cahnite by Palache and Bauer (1927) the mineral was
stated to be tetragonal, sphenoidal in symmetry. No form in a general position
was observed and the exact definition of the class was, therefore, not
possible. This ambiguity no longer exists and it can be stated, on the basis
of facts here presented, that cahnite belongs to the tetragonal disphenoidal
class,
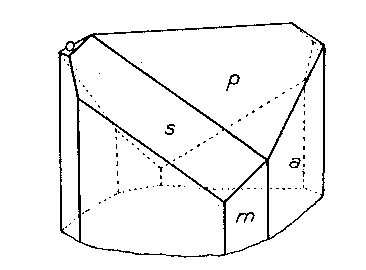
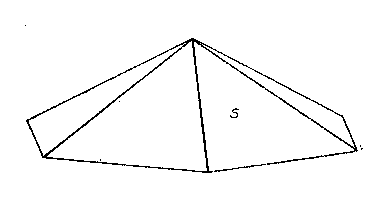
On his last visit to the Harvard Mineralogical Laboratory in 1935, Mr. Lazard Cahn brought with him a small specimen from Franklin, N. J., showing untwinned crystals of cahnite in small vugs in a mass of granular willemite. On these crystals Cahn had recognized the presence of a form modifying but two of the edges between {111} and {100}. A crystal was measured and the form was identified as {311}, with but two faces on the upper end of the crystal. This distribution of faces was confirmed on two other crystals and is shown in the accompanying figure.
The presence of this well-identified general form with the distribution of faces indicated leaves no doubt of the disphenoidal character of cahnite. It is a satisfaction to thus place on record the last scientific observation of Mr. Cahn upon the unusual mineral which he discovered and which bears his name.
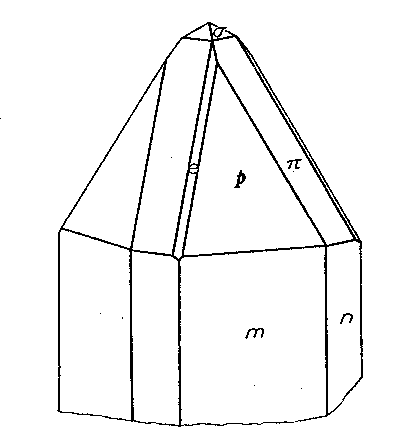
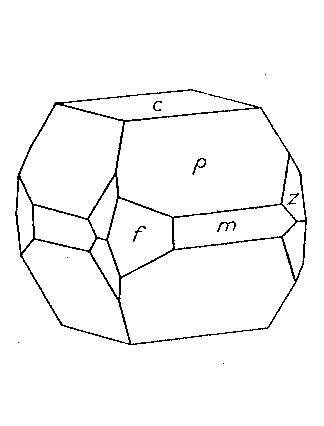
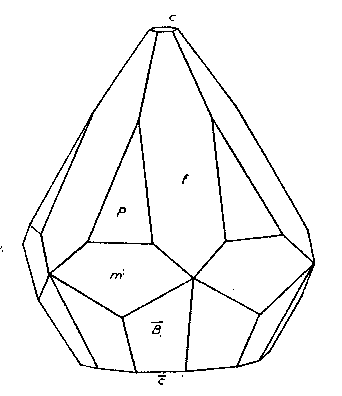
STOLZITE FROM ARIZONA AND NIGERIA Stolzite from Arizona Two localities for the rare tungstate of lead, stolzite, were found in 1939 by Mr. Edwin Over and specimens were secured for the Harvard Mineralogical Museum. The mineral is so rare that any new occurrence of it, especially when in well-formed crystals, seems worthy of record. Both localities are active tungsten mines. Primos Mine, near Dragoon, Arizona. The two specimens found were on the dump of the largest tunnel of the Boericke or Primos Mine. They come from a quartz vein which contains chalcopyrite, sphalerite, huebnerite, scheelite, fluorite and galena. In some cavities the sulphides had broken down and a coating of covellite was present. The stolzite crystals are in quartz vugs attached directly to the quartz. They are a millimeter or more in length and of a pale yellow color. The specific gravity was determined to be 8.26, the surest character by which they may be distinguished from wulfenite. These crystals, very few in number, are highly complex. One of exceptionally symmetrical development was measured and showed the forms: c{001}, n{130}, m(110}, e{011}, δ{113}, p{111}, r{441},*σ{137}, π{133} . Of these forms the more important are shown in Fig. 2, in which p {111} and π {133} are dominant. The prism zone is strongly developed, but the faces of the prism forms are broken and reflect poorly. The terminal forms are very brilliant and give consistent readings for position as shown in Table 1. One form, σ {137}, is new and is present with all four faces. Pough (1937) has reported this form on powellite from Tonapah, Nevada. The forms δ{113} and r {441} are weak forms and are not figured as they are minute on the crystal. {113} was first reported by Artini (1905) from Sardinia; {441} was found by Hodge-Smith (1934) to be characteristic of crystals of different habits from the South Mine, Broken Hill, Australia. The calculated angles of Table 1 are based on the elements of Hlawatsch (1897), which are confirmed by the results of this study. Reef Mine, Huachuca Mts., south of Tombstone, Arizona. The stolzite crystals are implanted on the walls of angular cavities in vein quartz from which sulphides have been weathered out. Elsewhere the vein contains scheelite, as well as galena, chalcopyrite, pyrite and limonite. The vein was originally worked for free gold, but the tungsten content is now the main value. The 1-2 mm. crystals are pale yellow and are of very varied habit due to the varying relative development of the forms: c{001}, *f{320}, m{111}, p{111} and *z{121}. Figures 3 and 4 show the two common variations; pseudocubic crystals are also rarely present. The new form {121} is well established by the measurements. It is known on wulfenite as is the more doubtful new prism {320}. The base {001} is rarely bright; more often it is dull and in some cases seems to have been etched to a hopper form. {111} is always very brilliant, as are the much smaller faces of {121}. The prism {320} is rounded as it so often is in wulfenite. Its nature, however, is easily apparent in the skew intersections it makes with the pyramid, and the few measurements obtained indicate a position about that indicated by the symbol.
TABLE 1. ANGLES OF STOLZITE
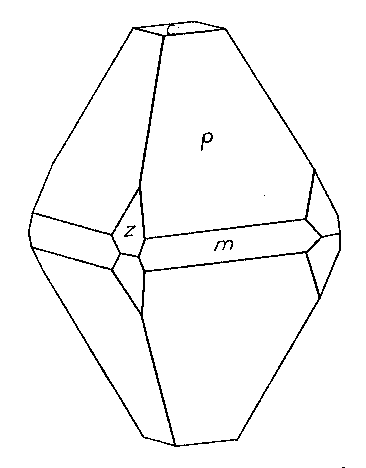
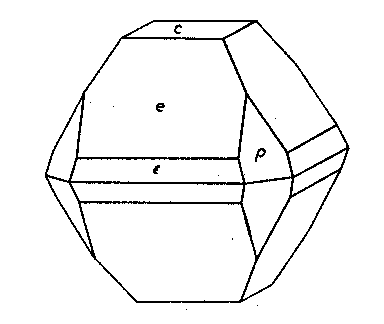
* New forms. Stolzite from Nigeria Ward's Natural Science Establishment of Rochester, New York, has recently supplied the Harvard collection with a sample of stolzite from the tin fields of Abuja, northern Nigeria. It is obviously a placer product, as the larger crystals, up to 3 mm. in diameter, are well rounded. But the smaller crystals, averaging about 1 mm. through, are, many of them, still sharp and brilliant. The color varies from lemon to orange-yellow and the sample seems to be very pure. The specific gravity of three separate crystals was determined on the Berman microbalance, giving an average value of 8.34±0.004, a value considerably higher than any hitherto published for this mineral. The calculated density is 8.45. Crystals were measured which showed the forms: c{001}, e(011}, ε{021}, p{111}, and π {133}. The base is dull, often very small and the crystals are pyramidal after either e or p {021} forms narrow truncations of the basal edges of e when it is dominant. Prisms seem to be absolutely lacking and it is indicated by faint lines of striation between e and p on one side only, the only visible evidence of hemihedrism. The figure shows a characteristic combination and habit.
NOTES ON ZINCITE Crystals
of zincite are so infrequent in the only important place of occurrence -
Franklin, N. J. - that any new find of them seems worthy of record
and particularly so when they occur in a new form. Some time ago Mr. L. H.
Bauer sent to the Harvard Mineralogical Laboratory a small specimen of ore
containing a vug lined with colorless fluorite and a few crystals of pale
yellow zincite. These were in part aggregated into spheroidal groups, but a
few stood isolated on the wall of the cavity. Measurement of one of these
millimeter-wide crystals showed it to present only the pyramid {10
ρ
ρ The fluorite crystals are also minute, clear and colorless and show a rare combination for this mineral, the forms present being d{011}, a{001} and m{113}. Minute amounts of fibrous white willemite and of a soft micaceous clay-mineral not more exactly determined, were also present. With this specimen Mr. Bauer also sent a superb specimen of very brilliant orange-colored zinc oxide, which he had received from Professor R. Norris Shreve of Purdue University. Professor Shreve, at the writer's request, secured the following statement as to the conditions under which these crystals were formed. It is written by Robert Ammon, Chief Metallurgist of the American Zinc Company of Illinois at St. Louis. In regard to the zincite crystals, the only information we have on these is that they were found in the combustion laboratory of a furnace operating on the production of French oxide. This furnace is oil fired, contains four carborundum retorts, each of which is fed with a supply of molten zinc in one end and the zinc vapor leaves the other end and is burned to ZnO. This furnace operates with an average temperature in the combustion laboratory of 1240° C. Our metallurgist at Hillsboro believes the combustion gases are oxidizing and the formation of these crystals starts with a small leak in the carborundum retort, through which a small amount of metallic zinc penetrates. His observation is that if a leak has been present for say 50 to 70 days, then, due to the exposure for so long a time at such a high temperature, this red crystal is formed.
The
crystals, which are very brilliant, are of varying habits. Most of them are
attached at the lower extremity and show on the free end a slender prism of
the first order together with the first order unit pyramid p {10 The following angles establish the nature of the forms found on these crystals:
New determinations of the density of zinc oxide were made with the microbalance on the material described below. Natural crystal fragments of zincite from Sterling Hill, N. J. (Berman, Am. Mineral., 12, 168, 1927) gave a density of 5.66±0.02 (4 determinations). The artificial zinc oxide of this paper gave a density of 5.665±0.02 (3 determinations). ULTRABASITE IDENTICAL WITH DIAPHORITE Ultrabasite was described by Rosický and Sterba-Böhm (1920) as an orthorhombic mineral with the composition Pb28Ag22Sb4Ge3S53. The few samples known came from the Himmelsfürst shaft at Freiberg, Saxony. In reviewing this work, Dr. Berman found that by transforming to a different unit cell, the forms and angles were essentially identical with those of diaphorite. Transformation, ultrabasite to diaphorite, 200/040/00½. The values are listed in the following table.
Parentheses
indicate that the form is not known on diaphorite. The specific gravity and other physical properties of the two minerals also agree. I wrote to Professor Rosický informing him of these facts and asked for type material for testing. A sample finally reached us with the statement that it yielded a spectroscopic evidence of the presence of germanium. The specimen was part of a crystal with all the appearance of diaphorite. The only visible impurity was a small amount of galena. A sample free from galena was examined by Mr. Kent, in the large two meter, quantitative grating spectrograph of this laboratory, and gave no trace of Ge, although a similar test on germanite gave the line of Ge. Under these circumstances one can only conclude that the analysis on which ultrabasite was defined must have been made on a different substance from that used for the morphologic and physical characters. The name, therefore can only be employed as a synonym for diaphorite. REFERENCES ARTINI, E., Rend. Inst. Lombard., Milan, 38, 574 (1905). HLAWATSCH, C., Zeits. Krist., 29, 130 (1897). HODGE-SMITH, T., Records Australian Mus., 19, 166 (1934). POUGH, F. H., Am. Mineral., 22, 57 (1937). ROSICKÝ AND STERBA-BÖHM, Zeits. Krist., 55, 430 (1920). NOTES * Contribution from the Department of Mineralogy and Petrography, Harvard University, No. 243.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||