| Home | AmMin | GMR | RiMG | Collectors Corner | Directory | Short Courses | |
|
|
|||||||

|
|
Volume 14, pages 188-196, 1929
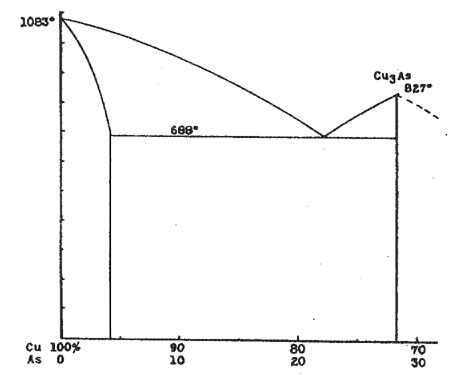
AN X-RAY STUDY OF THE DOMEYKITE GROUP* L. S. RAMSDELL, University of Michigan. Domeykite, algodonite and whitneyite are usually considered as definite minerals, and have been assigned the formulas Cu3As, Cu6As and Cu9As, respectively. However, various investigators have questioned the existence of these compounds, and have reported that microscopic examination reveals that these minerals are mixtures, rather than homogeneous compounds. In this present investigation, X-ray methods have been used to supplement the microscopic data, and rather conclusive information regarding these minerals has been obtained. THE COPPER-ARSENIC EQUILIBRIUM DIAGRAM The copper-arsenic equilibrium diagram has not been worked out completely. With high arsenic content the relations are complex, and the experimental difficulties become very great because of the volatility of the arsenic. However, the portion of the diagram which includes the compositions of domeykite and the others in this group is completely known.1 A definite compound, Cu3As, is formed, but there is no other compound between this and pure copper. About 4 per cent of arsenic can be present in solid solution in the copper. There is a eutectic with the composition Cu 78 per cent and As 22 per cent. Accordingly, in the range of compositions included in this investigation, it would be expected that the CuAs solid solution and the compound Cu3As would be the only two constituents present. With these as end members, any material of intermediate composition should consist of crystals of the predominating constituent embedded in a eutectic groundmass. The Cu-As diagram is shown in Figure 1. In this investigation the equilibrium diagram has been completely checked, both by microscopic and X-ray methods. Examinations were made of polished sections from a large number of samples which were prepared with varying compositions and by different methods. X-ray photographs were made of most of these preparations.
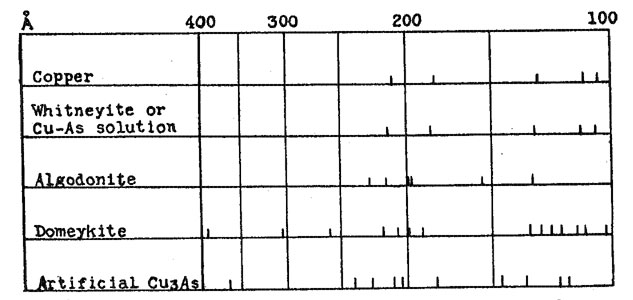
A few samples of Cu3As were prepared by passing arsenic vapor over hot copper, as described by Koenig.2 The crystals obtained in this manner gave X-ray patterns identical with those obtained by fusion, and no further study of these was made. All of the other samples were prepared by fusion, either of copper and arsenic, or of the natural minerals. Fusions were made in small crucibles, with a protective covering of borax. In some cases the borax was melted by heating from above before heat was applied to the sample, in others the material to be fused was dropped directly into molten borax. Very little volatilization of the arsenic occurred, and only with prolonged heating was the copper oxidized sufficiently to color the borax. Samples could be fused in this manner with a loss in weight of less than 0.1 per cent. In all cases the product obtained by fusion was such as would be predicted from the equilibrium diagram. The only constituents observed in polished sections were gray Cu3As and the Cu-As solid solution, which had a pale cream copper color. For the sake of brevity this will be referred to as the pink constituent. The relative proportions of the pink and the gray, and the amount of eutectic, varied with the composition. Different heat treatments; varying from slow cooling to quenching, affected the grain size, but nothing was obtained except the pink and the gray constituents. Likewise, only two types of X-ray diffraction patterns were secured from these preparations - that of Cu3As and of the Cu-As solid solution. These occurred either alone, or superimposed with differing relative intensities, depending upon the proportions of each constituent. The pattern from the Cu-As solution is almost identical with that of metallic copper, the only difference being that the spacings are slightly enlarged. This pattern and that of artificial Cu3As are shown diagramatically in Figure 2. It was expected that with such definite data regarding the Cu-As diagram, the correlation of these data with those obtained from the natural minerals would be quite simple, but this was not the case. Numerous polished sections of the natural minerals have been studied, analyses have been made in some cases, and all have been X-rayed. The data obtained by these various methods are in agreement among themselves, but in general are not what would be predicted on the basis of the equilibrium diagram. The results from each of the three minerals will be discussed separately.
WHITNEYITE Whitneyite is the only one of the three which gave results that can be directly correlated with the experimental data. Murdoch3 describes whitneyite as homogeneous, and apparently did not question the existence of a compound with the formula Cu9As. Borgström4 reports that whitneyite consists of a mixture of algodonite and copper, and denied the existence of a Cu9As compound. The data obtained in this investigation are essentially in agreement with those of Borgström. Two specimens of whitneyite were available for study. One was a portion of a large nodule from the Mohawk mine,5 and the other a large specimen from the Pewabic location, both in the Keeweenaw peninsula of Michigan. Numerous sections were cut off and polished. In every case the chemical analyses and X-ray photographs were made with material which had first been examined with the microscope. Both specimens showed the same general characteristics. They were somewhat malleable, and fractured with great difficulty. The fracture surface was fine granular, and appeared homogeneous. On polished surfaces lack of homogeniety was apparent, sometimes even to the unaided eye. Two constituents were present, one pink and the other cream in color. The pink was similar to the Cu-As solution in appearance, and was identified as such both by the chemical analyses and the X-ray patterns. This identification was not difficult, because the pink constituent occurred in rather large areas, with very little admixture of the cream. Thin slices of the material were polished on both sides, after which the comparatively pure pink areas could be separated very readily. The cream constituent, however, was usually intimately mixed with the pink, and could not be obtained in pure form. The X-ray pattern from the pink constituent was similar to that from the Cu-As solution. The cream, mixed with the pink, gives this same pattern, together with the stronger lines of the pattern which was obtained from the algodonite specimens. Thus the X-ray evidence identifies the pink constituent as being the Cu-As solid solution, and the cream as algodonite. In the chemical analysis, the arsenic was determined as Mg2As2O7, while the copper was determined electrolytically in alkaline solution; under such conditions traces of Ni or Co would also be deposited. However, a qualitative test for Ni was negative. In one specimen there were large cavities and stringers of some foreign material, while in the other the impurities were found in minute cavities. The samples were freed from these as far as possible, and the following results were obtained: PINK CONSTITUENT
No attempt was made to determine the remaining 1.20 per cent of the impurities. Recalculated on the basis of 100 per cent, the proportions would be: copper 94.8 per cent and arsenic 5.2 per cent. This agrees very well with the values of copper 96 per cent and arsenic 4 per cent for the Cu-As solid solution, for there was present a slight amount of the cream constituent, with its higher arsenic content. An analysis was also made on a specimen which appeared homogeneous to the eye, but which was clearly seen to be an intimate mixture of cream and pink when examined with the microscope. This gave results very close to the theoretical percentages for a compound with the formula Cu9As. It may be that there is a eutectic between natural Cu6As and the Cu-As solution, in which the proportions of copper and arsenic happen to correspond approximately to this theoretical ratio. An analysis of a specimen containing areas of such a eutectic might very readily be misinterpreted as indicating a definite compound, especially when it is remembered that the early analyses were made long before the development of a technique for examining opaque minerals with the microscope. The original analyses were made by Genth6 in 1859, on material which he stated was selected with great care, and which was apparently quite pure. A second group of analyses by Genth was made on material noticeably unhomogeneous, containing visible algodonite. It was assumed that a good separation of the whitneyite from the algodonite had been obtained because the analyses checked with the earlier ones. Thus an analysis on material of questionable homogeniety was made the criterion for further separations. The analyses of Koenig7 on material which he called semi-whitneyite, with copper about 94 per cent and arsenic 6 per cent, would indicate that his samples corresponded to the Cu-As solid solution. ALGODONITE Murdoch8 describes specimens of algodonite as consisting of a mixture of unknown cream and gray constituents, with the cream in slight excess, and concludes that algodonite is therefore not a definite compound. He also assumes that algodonite and domeykite are similar. Two large specimens of algodonite were available for this investigation. One came from the Mohawk mine, and the other was found as "float" in Baraga County, Michigan. Both had a fine grained fracture and steel gray color. They are more easily fractured than the whitneyite specimens, but much less so than domeykite, which can be crushed quite easily. Polished sections from these two specimens showed a homogeneous material of cream color, similar to the cream constituent of the whitneyite.. There were small inclusions of quartz, a few small blebs of a pink material which appeared to be copper, and the Mohawk specimen showed small patches of an unknown gray substance. An insufficient amount of this material was present to make it possible to obtain an X-ray pattern. These inclusions were present in such small amounts that there seemed to be no reason to regard them as an essential part of the material, nor to regard algodonite as anything but a homogeneous substance. The X-ray pattern from algodonite is entirely different from the patterns obtained from the artificial preparations. The Cu-As diagram would indicate that anything with a composition corresponding to Cu6As should be a mixture of Cu9As and the Cu-As solution in about equal proportions. As already indicated, the microscopic evidence was to the contrary, and the X-ray pattern likewise indicates that algodonite is a naturally occurring compound, Cu6As in composition, which is not duplicated in artificial preparations. Its pattern shows none of the lines characteristic of either artificial Cu9As or the Cu-As solution. This is shown in Figure 2. The relation of this natural compound to the artificial material is shown by its behavior upon heating. When fused and cooled, this homogeneous compound breaks up into Cu3As and Cu-As solution, just as would be predicted from the diagram. Actual fusion is not necessary. When heated to a point somewhat below the fusion point, the decomposition occurs and the pink and gray constituents are formed. This is in agreement with Borgstrom,9 who reported that algodonite is unstable at its melting point, and changes to copper and Cu3As. DOMEYKITE Murdoch9 describes domeykite as a mixture. Three specimens examined by him were composed of unknown cream and gray constitutents, identical with algodonite, while a fourth showed cream and purple constituents. No such results were found in the specimens examined in this investigation. Physically the domeykite is quite different from algodonite, and except for small inclusions, the polished sections seemed to be homogeneous. The constancy of the analyses and the identical X-ray patterns of specimens from widely different localities argue against domeykite being a mixture, for in such a case it would be expected that the proportions of the two constituents would vary. Likewise, if domeykite were a mixture with an average composition of Cu3As, one component would have to be higher, and one lower in copper, than the compound Cu3As itself. But the X-ray pattern shows no lines corresponding to the Cu-As solution, or to algodonite; nor to the artificial compound with lower copper content which was obtained in some of the preparations, and which was probably Cu5As2. If domeykite is a mixture, it must be composed of compounds different from any of these. The X-ray pattern of domeykite is not only different from the compounds with higher and lower content of copper, but is also different from that of artificial Cu3As; which indicates that the compound Cu3As is dimorphous. Both forms are stable at ordinary temperatures, and there is a monotropic inversion from domeykite to the artificial form at a temperature close to the melting point of domeykite. If natural domeykite is fused, or heated nearly to the fusion point, it inverts to the other form, and does not change back upon cooling. This change is shown by the X-ray patterns, and also by the microscope. The cream colored domeykite changes to gray, and there is formed a small amount of the Cu-As solid solution, occurring as interstitial material between the grains of gray Cu3As. This does not mean that domeykite contains excess copper over that required by the formula Cu3As, but rather is an illustration of the fact that the artificial compound always seems to be slightly high in arsenic. As previously stated, the crystals of Cu3As prepared by passing arsenic vapor over hot copper are identical with the material prepared by fusion. Accordingly the crystallographic data, as determined by Wright11 on crystals prepared in this manner, are not applicable to domeykite. In this investigation, however, no new data for domeykite were obtained. As far as its behavior in reflected polarized light is concerned, domeykite is isotropic, but its X-ray diffraction pattern does not agree with any simple cubic structure. A single X-ray photograph was taken on a sample of mohawkite, and the pattern secured was indistinguishable from that of domeykite. No further study was made, but it seems probable that its character is quite analogous to that of domeykite. SUMMARY 1. According to the copper-arsenic constitution diagram, the only compound in the range from Cu 70 per cent-As 30 per cent to pure copper is Cu3As. Copper may contain 4 per cent of arsenic in solid solution. 2. A series of artificial preparations, made chiefly by fusion of copper and arsenic in proper proportions, was in entire agreement with the diagram, as shown by microscopic examination and X-ray photographs. No constituents but Cu3As and Cu-As solution were found. 3. Whitneyite, Cu9As, is not a definite compound, but a mixture of algodonite and Cu-As solution. 4. Algodonite, Cu3As, is a definite compound, but is unstable at its melting point, breaking down into Cu3As and Cu-As solution, and therefore cannot be prepared artificially by fusion. 5. Domeykite, Cu3As, is a definite compound, also unstable at its melting point. At this temperature there is a monotropic inversion to a dimorphous form, which corresponds to the Cu3As compound formed in artificial preparations. While this article was in press, there appeared a paper "ALGODONITE AND WHITNEYITE" by F. Machatschki in Neues Jahrb. Mineral., Bl. Bd., LIX, Abt. A., 137-158 (1929). This new investigation confirms the conclusion that there is no Cu9As compound. The specimen of whitneyite studied showed 60% of the Cu-As solution and 40% of a substance with a hexagonal structure. A specimen of algodonite had the same constituents in the proportion of 20 and 80 per cent, respectively. In my investigation only three well defined lines were present on the algodonite films, and no attempt was made to determine the structure. I find that these three lines, together with four other very doubtful ones agree with those obtained by Machatschki on his films. The structure he derives is hexagonal close-packed, with two atoms in the unit cell. The Cu and the As atoms are assumed to be equivalent, and are distributed in the ratio of 5 to 1. It is suggested that possibly there is a range of compositions in which this structure is stable. This is quite possible, but his conclusion that the hexagonal compound is Cu5As rather that Cu6As does not seem justified. Instead of the obvious conclusion that his specimen was a mixture of algodonite (Cu6As) and the Cu-As solution, he assumes that it is typical algodonite, and that this mixture has a composition corresponding to Cu6As. Since 20% of the mixture is Cu-As solution, with Cu 95% and As 5%, he figures that the remainder must have Cu and As in the ratio of 4.9:1. His conclusion is that the hexagonal compound is Cu5As. It would seem that such a conclusion should be based on an actual analysis of the specimen used. Algodonite does occur in comparatively pure form, and an analysis of such material in my investigation gave Cu 83.2% (theoretical for Cu6As is Cu 83.5%.) NOTES * Paper presented at the ninth annual meeting of the Mineralogical Society of America at New York City, December 28, 1928. 1 K. Friedrich. Metallurgie, 5, 529, (1908). 2 Zeil. Krist., 38, 532, (1904). 3 J. Murdoch. Microscopic Determination of the Opaque Minerals, p. 74. 4 L. H. Borgström. Geol. För. Förh., 38, (1916); Am. Mineral., 4, 91 (1919). 5 The writer is indebted to Mr. F. F. Bradley, of Toledo, O., for this specimen of whitneyite. 6 F. A. Genth. Am. Journ. of Science, 27,400 (1859); 33,191 (1862). 7 C. A. Koenig. Zeit. Krist., 38, 537 (1904) 8 Murdock, loc. cit., p. 37. 9 loc. cit. 10 loc. cit., p. 37. 11 F. E. Wright. Zeit. Krist., 38, 551 (1904).
|