| Home | AmMin | GMR | RiMG | Collectors Corner | Directory | Short Courses | |
|
|
|||||||

|
|
Volume 53, pages 300-305, 1968
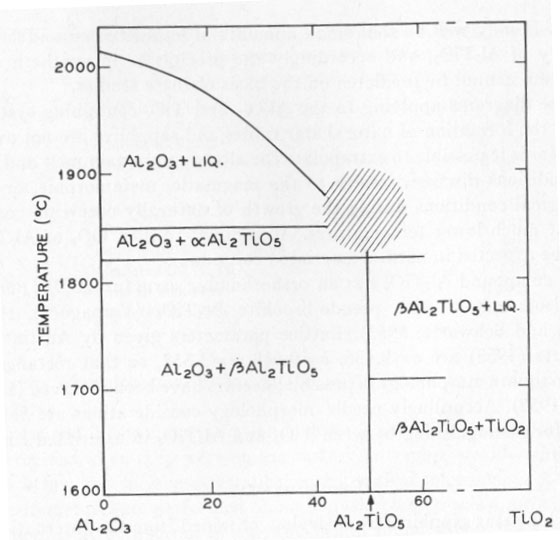
ON THE CAUSE OF ASTERISM IN STAR CORUNDUM K. NASSAU, Bell Telephone Laboratories, Inc. Murray Hill, New Jersey. INTRODUCTION It has been generally accepted that asterism in corundum (star rubies and sapphires) is caused by needles or rutile (TiO2) aligned along crystallographic planes of the corundum (e.g. Webster, 1962). The evidence given by Tait (1955) was: (1) the presence of titanium, (2) the needlelike habit, and (3) the square cross-section of the needles. Based on the analogy with rutilated quartz, and by eliminating octahedrite and brookite by the morphology, rutile was accordingly identified. This type of evidence, however, is explained equally well by either TiO2 or the compound aluminum titanate Al2TiO5. CORUNDUM GROWTH CONSIDERATIONS The technique for the laboratory growth of star rubies and sapphire has been revealed in patents by the Linde Division of the Union Carbide Corp. (Burdick et al., 1949, 1954). Corundum is grown by the Verneuil technique with the addition of, preferably, 0.1 to 0.3 percent titanium oxide in the feed powder. The corundum as grown is clear, but develops asterism upon heat treatment at 1100 to 1500°C (Webster, 1962; Burdick et al., 1949, 1954) due to precipitation of the excess titanium, the solubility of which is exceeded at this temperature. The phase diagram of the Al2O3: TiO2 system has been studied by Bunting (1933) and by Lang, Fillmore and Maxwell (1952). The relevant parts of these studies are included in Figure 1. There is a slight uncertainty as to whether Al2TiO5 melts congruently or incongruently near 1860°C in the shaded region, but this does not affect the present discussion. Similarly, there may be other forms of Al2TiO5 besides the α form which is stable above 1820°C, and the β form stable below 1820°C. According to this phase diagram, Al2TiO5 is the phase that should separate out from the corundum when the solubility is exceeded at temperatures near the melting point of corundum.
Confusion, however, enters in the temperature region below 1600°C. According to Lang, Fillmore and Maxwell (1952), ß-Al2TiO5 becomes unstable, dissociating into Al2O3+TiO2 again at lower temperatures. They reported 90 to 98 percent Al2TiO5 at 1600°C, and only 2 to 10 percent Al2TiO5 at 1200-1350°C. Hamelin (1951) similarly states that in the presence of 5 percent boric acid at 1250°C no Al2TiO5 is formed after 1 hour, but complete conversion was obtained at 1450°C in 10 minutes. Based on these results the asterism in synthetic corundum would be expected to originate from TiO2. In a study on the solubility of TiO2 in Al2O3, Winkler, Sarver and Cutler (1966) however prepared Al2TiO5 by firing at 1400°C for 2 hours and obtained Al2TiO5 with no unreacted Al2O3 or TiO2. Vamaguchi (1944) also obtained Al2TiO51 at 1400°C. A quick check, heating an intimate mixture of Al2O3 and TiO2 at 1300°C for 3 hours, produced essentially no Al2TiO5, consistent with Lang et al., (1952). It may well be that small amounts of impurities can affect the stability of Al2TiO5, and accordingly the precipitate in synthetic star corundum cannot be predicted on the basis of these studies. Phase diagrams applying to the Al2O3- and TiO2-containing systems during the formation of natural star rubies and sapphires are not available. Nor is it possible to extrapolate the already uncertain melt and sinter conditions discussed above to the magmatic, metamorphic, or hydrothermal conditions during the growth of naturally occurring corundum at much lower temperatures. Accordingly, either TiO2 or Al2TiO5 could be expected in natural asteriated corundum. The compound Al2TiO5 has an orthorhombic structure (8,9) (Bbmm, Vh17) isomorphous with pseudo-brookite Al2TiO5 (Yamaguchi, 1944; Austin and Schwartz, 1953). Lattice parameters given by Austin and Schwartz (1953) are a=9.436, b=9.648, c=3.557, so that rectangular or near-square morphology is possible; needles have been observed (Lang et al., 1952). Accordingly needle morphology considerations are inadequate for distinguishing between TiO2 and Al2TiO5 in asteriated corundum. EXPERIMENTAL Pale blue star sapphires from Ceylon, obtained from the International Gem Corp., New York, and synthetic star ruby from the Linde Company, were examined by optical microscopy, electron microscopy, and by electron probe microanalysis. Optical examination showed the typical arrangement of three sets of needle-like inclusions in the basal plane as illustrated by Tait (1955). The needles were square to rectangular in cross-section; in some cases they appeared to be empty (hollow). A number of irregularly shaped and placed inclusions were also observed in the natural material, but were not further investigated. Needle diameters ranged from a few tens of microns in diameter down. In the synthetic material the needles were much smaller and could not be used for further examination. Attempts were made to take plastic replicas from polished and fractured surfaces for electron microscopy in the hope of pulling out a needle and obtaining electron diffraction patterns. A number of attempts did not yield any usable replicas. Electron probe microanalysis was performed on a Cambridge Microscan instrument. The sapphire samples were polished on diamond powder on polystyrene, ending up with 1/4-µ diamond, vapor coated with carbon in vacuum, and analyzed for the AlKα1 line by use of a gypsum crystal and for the TiKα1 line by using a lithium fluoride crystal. A check was also run across the whole spectral range of the instrument but no other elements were detected, either in the inclusions or in the bulk of the sapphires. TABLE 1. ELECTRON BEAM MICROANALYSIS CONCENTRATIONS AS INTENSITY RATIOS COMPARED TO THE PURE METAL
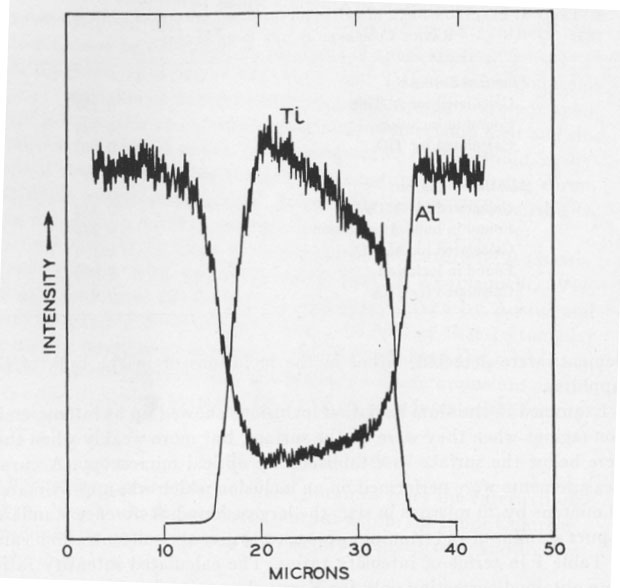
Examined in titanium radiation inclusions showed up as intense emission regions when they were on the surface, but more weakly when they were below the surface as established by optical microscopy. Accurate measurements were performed on an inclusion which was approximately 30 microns by 20 microns in size, the largest found. Reference standards of pure aluminum and titanium were used to give the concentration value of Table 1 in terms of intensity ratios. The calculated intensity ratios were obtained correcting only for X-ray absorption (Birks, 1963). Fluorescence and atomic number corrections are expected to be of minor importance. DISCUSSION In the electron probe microanalysis of small, thin specimens there is the danger of spurious readings from picking up radiation from the matrix above, to the sides, or from below the specimen. Optical examination showed that there was no corundum overlaying the inclusion. The approximately flat regions obtained in Figure 2 confirm that the surrounding corundum was not contributing. The slight slope, however, indicated that the inclusion was probably quite thin and varying in thickness, so that the titanium reading obtained would be on the low side. Accordingly the values of Table 1 clearly rule out the possibility of Al2TiO5 and confirm TiO2. The Al reading from the inclusion is expected to be on the low side due to the strong absorption of the Al radiation by the TiO2. Since the inclusion is thin, some Al radiation is expected to be observed even from TiO2. The inclusions in the synthetic ruby available were found to be too small for analysis, but on the basis of the phase diagram considerations discussed could be either TiO2 or Al2TiO5. The possibility remains that natural star corundum which grew under different conditions from the sample here examined could contain Al2TiO5 needles. ACKNOWLEDGMENTS The author is grateful for experimental assistance by Miss S. E. Koonce, H. Scheiber, and particularly Mrs. I. D. Payne for the electron probe analysis work.
REFERENCES AUSTIN, A. E. AND C. M. SCHWARTZ (1953) Acta Crystallogr. 6,812-13. BIRKS, L. S. (1963) Electron Probe Microanalysis. Interscience Publishers, New York, p. 125 ff. BUNTING, E. N. (1933) J. Res. U. S. Nat. Bur. Stand. 11, 719-725. BURDICK, J. N. AND J. W. GLENN (1949) U. S. Pat. 2,488,507 Nov. 15, 1949. __________ AND R. A. JONES (1954) U. S. Pat. 2,690,062, Sept. 28, 1954. HAMELIN, M. (1951) Bull. Ceram. Soc. (France) 11,14-16. LANG, S. M., C. L. FILMORE AND L. H. MAXWELL (1952) J. Res. U. S. Nat. Bur. Stand. 48, 298-312. TAIT, A. S. (1955) J. Gemology 5, 65-72. WEBSTER, R. (1962) Gems, Vol. 1, Butterworths, Washington, D. C., p. 307. WINKLER, E. R., J. F. SARVER AND I. B. CUTLER J. Amer. Ceram. Soc. 49, 634-637. YAMAGUCHI, G. (1944) J. Japan Ceram. Assn. 52, 6-7. NOTE 1 Yamaguchi gave the name tieilite to ß-Al2TiO5, but did not observe it in nature.
|